Rapid PNGase F by SDS-PAGE Protocol (P0710)
- Prepare 3X reducing SDS loading buffer (4 µl of 1.25 M DTT, 130 µl 3X SDS Loading Buffer; (NEB #B7703S).
- Add 5 µl of 3X reducing SDS loading buffer per 10 µl of sample (containing 2-3 µg of glycoprotein, for instance IgG)*.
- Incubate at 94°C for 5 min, cool down.
- Load sample (load control samples side-by-side) and protein marker (NEB #P7703S) on a 10–20% Tris–Glycine gel. Run gel at 130-200 V. Remove the gel from the cast and place it in a plastic tray with Coomassie Blue stain solution (follow manufacturer recommendations). Record images using a white light trans-illuminator or scanner.
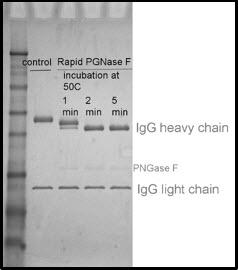
The picture below illustrates how IgG (heavy chain) migrates to a lower position after deglycosylation. Treatment with Rapid PNGase F for 1 minute results in only partial deglycosylation (intermediate shifts). Treatments of 2 to 5 minutes have a complete shift in molecular weight, indicating that the reaction has reached completion and the glycoprotein is fully deglycosylated.
* Typically, IgG gel shifts are most visible when sample does not exceed 3ug of protein per lane. For other glycoproteins, with high heterogeneity, the optimal load has to be determined experimentally.

