Standard RNA Synthesis (E2050)
Protocols.io also provides an interactive version of this protocol where you can discover and share optimizations with the research community.
We strongly recommend wearing gloves and using nuclease-free tubes and reagents to avoid RNase contamination. Reactions are typically 20 μl but can be scaled up as needed. Reactions should be assembled in nuclease-free microfuge tubes or PCR strip tubes.- Thaw the necessary kit components, mix and pulse-spin in microfuge to collect solutions to the bottoms of tubes. Keep on ice.
- Assemble the reaction at room temperature in the following order:
REAGENT AMOUNT FINAL CONCENTRATION Nuclease-free water X µl NTP Buffer Mix 10 µl
10 mM each NTP final
Template DNA X µl
1 µg
DTT (0.1M)*
1 µl
5 mM
T7 RNA Polymerase Mix 2 µl Total reaction volume 20 µl
*Addition of DTT (5mM final) to the reaction is optional but recommended.The RNA polymerase in the kit is sensitive to oxidation and could result in lower RNA yield over time due to repeated handling etc. Adding DTT to the reaction may help restore the kit performance in such cases. Adding DTT will not compromise the reaction in any situation.
- Mix thoroughly and pulse-spin in a microfuge. Incubate at 37°C for 2 hours.
Reaction time depends on template amount, quality and RNA transcript length. For reactions with transcripts longer than 0.3 kb, 2 hour incubation should give you the maximum yield. For reaction times of 60 minutes or less, a water bath or heating block may be used; for reaction times longer than 60 minutes, we recommend using a dry air incubator or a thermocycler to prevent evaporation of the sample.
For reactions with short RNA transcripts (< 0.3 kb), we recommend an incubation time of 4 hours or longer. It is safe to incubate the reaction for 16 hours (overnight). For example, we have achieved good yield with only 0.2 μg plasmid template encoding a 50-mer RNA by incubating the reaction overnight at 37°C.
Reaction set up for short transcripts (< 0.3 kb):
REAGENT AMOUNT FINAL CONCENTRATION Nuclease-free water X µl NTP Buffer Mix 10 µl
6.7 mM each NTP final
Template DNA X µl
1 µg
DTT (o.1M)*
! μl
5 mM
T7 RNA Polymerase Mix 2 µl Total reaction volume 30 µl
*Addition of DTT (5mM final) to the reaction is optional but recommended.The RNA polymerase in the kit is sensitive to oxidation and could result in lower RNA yield over time due to repeated handling etc. Adding DTT to the reaction may help restore the kit performance in such cases. Adding DTT will not compromise the reaction in any situation.
Compared to the standard reaction, this reaction uses 10 μl more water. The volume of NTP Buffer Mix and T7 RNA Polymerase Mix, however, remains the same. The kit contains sufficient materials for 50 reactions.
Note that the amount of NTP Buffer Mix in a standard 20 μl reaction can vary from 2 to 10 μl. The final yield is proportional to the amount of input nucleotides, meaning that the nucleotide incorporation efficiency remains the same when different amounts of NTP are used. Figure 1 shows the time course of standard RNA synthesis from 1 μg control DNA template coding for a 1.8 kb RNA transcript with the HiScribe T7 Quick Kit using 10 μl and 5 μl NTP Buffer Mix in a 20 μl reaction.
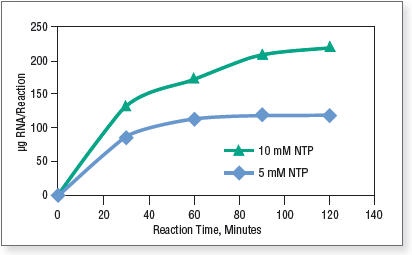
Figure 1. RNA synthesis with different amounts of NTP.
Reactions were incubated at 37°C in a thermocycler. Transcripts were purified by spin columns and quantified on a NanoDrop™ Spectrophotometer. - Optional: DNase treatment to remove DNA template. Standard reactions
are capable of generating large amounts of RNA, at concentrations up to
10 mg/ml. As a result, the reaction mixture is quite viscous. It is easier to
perform DNase treatment after the reaction mixture is diluted. To remove
template DNA, add 30 μl nuclease-free water to each 20 μl reaction, followed
by 2 μl of DNase I (RNase-free), mix and incubate for 15 minutes
at 37°C.
- Proceed with purification synthesized RNA (we recommend the Monarch RNA Cleanup Kits, NEB #T2040 or #T2050) or analysis of transcription products by gel electrophoresis.

