Isolation of mRNA from Mammalian Cells Using the Magnetic mRNA Isolation Kit (NEB #S1550)
Isolation Preparation
Allow all kit components to come to room temperature.
Resuspend Oligo d(T)25 beads by agitating at room temperature (RT) for 30 minutes.
Reuse Oligo d(T)25 beads for second-round poly(A)+ selection.
Oligo d(T)25 beads can be re-used for a second-round of purification of a poly(A)+ RNA eluent without regeneration. Wash beads once with an additional 100 μl of elution buffer. Place in magnetic rack and pull beads to the side of the tube. Remove and discard wash solution. Wash beads once with 200 μl of Lysis/Binding Buffer then re-apply previously isolated eluent to beads after adjusting salt concentration to 0.5 M LiCl or NaCl. Repeat isolation steps.
Regeneration of Oligo d(T)25 beads.
Oligo d(T)25 beads can be regenerated and used to isolate RNA from a different source. Add 0.1 N NaOH to the beads and incubate at room temperature with agitation for 5 minutes. Wash the beads three times with elution buffer (or sterile RNase-free H2O), equilibrate the beads by washing three times with sterile RNase-free 1X PBS (pH 7.4) containing 0.1% Tween 20 and store in sterile RNase-free 1X PBS (pH 7.4) containing 0.1 % Tween 20 and 0.05% NaN3.
Isolation of mRNA from Mammalian Cells:
Precautions should be taken to avoid ribonuclease contamination during the isolation procedure. Clean the area where isolation will be performed with RNase decontamination reagent before starting the isolation.
Wear latex gloves or equivalent at all times when handling kit components.
Step 1
Aliquot the appropriate volume of Oligo d(T)25 beads for the scale of isolation (Table 1).
Add 200 μl of Lysis/Binding Buffer to beads, vortex briefly and mix with agitation for 2 minutes. Beads should remain in the lysis/binding wash solution until removal immediately before adding the cell lysate.
Step 2
Adherent Cells
1. Aspirate media from cell culture plate and wash once with 1X PBS (pH 7.4).
2. Add the appropriate volume of Lysis/Binding Buffer to cells and gently swirl by hand.
Suspension Cells
1. Pellet cells by centrifuging at 1,000 rpm for 5 minutes at 4oC.
2. Aspirate media and was once with cold sterile 1X PBS (pH 7.4).
3. Pellet again, discard PBS and add the appropriate volume of Lysis/Binding Buffer to cells.
4. Agitate to suspend cells in Lysis/Binding Buffer.
Step 3
Incubate at RT for 5 minutes with gentle agitation. If the solution is viscous, pass the lysate several times through a 21-gauge needle attached to a 1 or 2-ml syringe. A noticeable decrease in viscosity should be observed.
Place the microcentrifuge tube containing the beads and lysis/binding wash into the magnetic rack and pull the magnetic beads to the side of the tube.
Step 4
Remove Lysis/Binding wash and add the cell lysate to the equilibrated magnetic beads.
Place cell lysate-and-bead suspension on the agitator and incubate at RT for 10 minutes.
Place microcentrifuge tube into the magnetic rack and pull magnetic beads to the side of the tube, remove and discard supernatant.
Step 5
Add the appropriate volume of Wash Buffer 1 (Table 1) to the beads and mix with agitation for 1 minute.
Place microcentrifuge tube into magnetic rack and pull magnetic beads to the side of the tube, remove and discard wash solution.
Repeat once.
Step 6
Add the appropriate volume of Wash Buffer 2 (Table 1) to the beads and mix with agitation for 1 minute.
Place microcentrifuge tube into the magnetic rack and pull magnetic beads to the side of the tube, remove and discard wash solution.
Repeat once.
Step 7
Add the appropriate volume of Low Salt Buffer (Table 1) to the beads and mix with agitation for 1 minute.
Place microcentrifuge tube in the magnetic rack and pull magnetic beads to the side of the tube, remove and discard wash solution.
Step 8
Add the appropriate volume of Elution Buffer (Table 1) and vortex gently to suspend beads.
Incubate at 50oC for 2 minutes with occasional agitation to elute poly(A)+ RNA. >90% of the poly(A)+ RNA bound to the beads is recovered in this step.
Place microcentrifuge tube in the magnetic rack and pull the magnetic beads to the side of the tube. Transfer eluent to a clean, sterile RNase-free tube and store on ice for immediate quantitation, or place at -80oC for long-term storage.
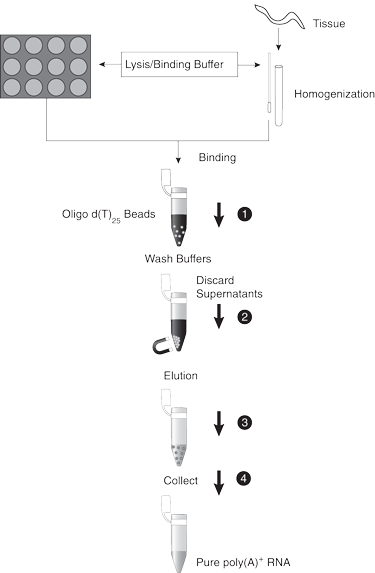
Table 1: Recommended volumes for different isolation scales (for cells).
| NUMBER OF CELLS | VOLUME OF OLIGO D(T)25 BEADS | VOLUME OF LYSIS/BINDING BUFFER | VOLUME OF WASH BUFFERS & LOW SALT BUFFER | VOLUME OF ELUTION BUFFER | EXPECTED YIELD (µg) |
|---|---|---|---|---|---|
| 5 x 104 | 50 µl | 250 µl | 250 µl | 50 µl | ~0.5–2 |
| 1 x 105 | 100 µl | 500 µl | 500 µl | 100 µl | ~1–3 |
| 5 x 105 | 100 µl | 500 µ | 500 µl | 100 µl | ~2–4 |

