What is the difference between intron-encoded and retroviral reverse transcriptases?
Posted on Wednesday, November 16, 2022
By
Topic: Tips for the lab, What is Trending in Science
At the time reverse transcriptases (RTs) were discovered in the 1970s, the central dogma of molecular biology stated that information only flows from DNA to RNA to protein. Researchers investigating and theorizing the existence of an enzyme that could copy RNA into DNA, contrary to the central dogma, were initially met with skepticism. However, in the 50 years since the Nobel Prize-winning discovery of RTs, the ability to synthesize DNA from an RNA template has enabled researchers to study RNA using the same molecular approaches as DNA investigations. Typically, reverse transcription is coupled with methods such as PCR, qPCR, or loop-mediated isothermal amplification (LAMP) for further downstream analysis.
The most common and well-characterized RTs are derived from retroviruses. Many commercially-available retroviral RTs have been genetically engineered with mutations that confer specific attributes, such as reduced RNase H activity, increased half-life and increased thermostability. A second family of RTs, distinct in sequence and domain organization, is found within the intron-encoded proteins of group II introns. Group II intron-encoded RTs are commonly found in bacteria, archaea and eukaryotic organelles.
Both retroviral RTs and group II intron-encoded RTs can make complementary DNA (cDNA). They share some conserved domains; both have RNA-directed DNA polymerase activity and DNA-directed DNA polymerase activity. A typical retroviral RT also contains an RNase H domain at the C terminus, and therefore has RNase H activity and can degrade the parental RNA strand in the DNA-RNA hybrid. An intron-encoded RT lacks this domain and activity.
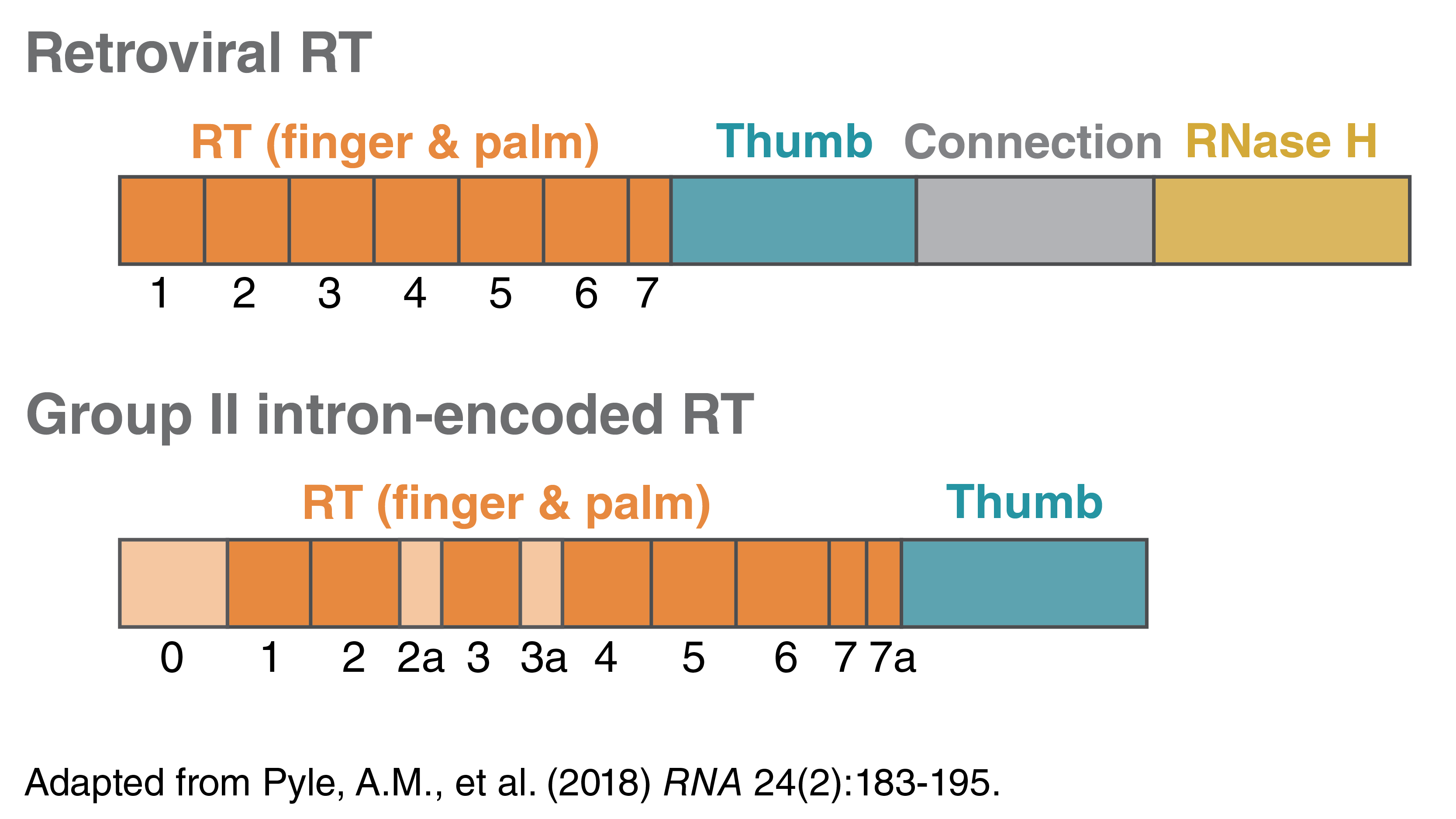
A typical retroviral RT contains a polymerase domain with several conserved motifs at the N-terminus and the RNase H domain at the C-terminus. Group II intron-encoded RTs, similar to retroviral RTs, contains several conserved motifs within the RT (motifs 1-7, orange). The N-terminal extension (0) and insertions between the conserved sequence blocks (motifs 2a, 3a, and 7a, tan) are observed in group II intron RT but not in retroviral RTs. The intron-encoded RTs often do not have the RNase H domain and therefore do not have the RNase H activity.
New England Biolab's current RT portfolio includes several retroviral RTs, including the thermostable LunaScript® RT Supermix (NEB #M3010) and WarmStart® RTx, Reverse Transcriptase (NEB #M0380) as well as AMV Reverse Transcriptase (NEB #M0277), M-MuLV Reverse Transcriptase (NEB #M0253), and ProtoScript® II Reverse Transcriptase (NEB #E6560). Retroviral RTs are widely available and have been incorporated into many established protocols.
We have recently added our first group II intron-encoded RT to our portfolio, Induro™ Reverse Transcriptase (NEB #M0681) (Induro stands for Intron-Encoded Endurance RT). While group II intron-encoded RTs do not replace retroviral RTs, they do offer some advantages, specifically when it comes to challenging RT reactions such as full-length cDNA synthesis from long transcripts. Induro RT demonstrates high processivity, is thermostable, tolerates a wide range of common inhibitors found in RNA samples, and can bypass modified nucleotides.
(1) High processivity is required for long cDNA synthesis
Long transcripts can be challenging to reverse transcribe, but the high processivity of group II intron-encoded RTs ensures success in preserving the complete information present in the RNA molecule. A non-processive RT will produce more truncated cDNA transcripts. This makes it difficult to get an accurate picture of isoform abundance in a long transcript.
Processivity refers to the ability to incorporate many nucleotides into the cDNA molecule during a single template binding event. It also applies to how tightly bound the RT is to its template, aiding in its ability to overcome inhibitors that may be present in the RNA sample.
Intron-encoded RTs have higher processivity than retroviral RTs. Induro RT can synthesize cDNA at least 14 kb in length in as little as 10 minutes. Retroviral RTs have a very high turnover rate, so long DNA can be made if there is sufficient enzyme and adequate incubation time. Still, the ability of retroviral RTs to synthesize challenging full-length cDNAs from long transcripts is limited. However, for general cDNA synthesis (up to roughly 8 kb), retroviral RTs are still the recommended option.
(2) A thermostable RT helps with difficult secondary structures
Long transcripts typically have more complex, folded secondary or tertiary structures. A higher reaction temperature can facilitate opening up these structures. A thermostable RT can maintain enzymatic activity at elevated temperatures. Induro RT can generate cDNA at temperatures as high as 60°C, with an optimal temperature of 55°C.
(3) Better inhibitor tolerance gives a higher cDNA yield
Inhibitors in RNA samples can carry over from the extraction and purification. Some common inhibitors include KCl, NaCl, MgCl2, formalin, paraffin, Tween®20, Igepal, ethanol, isopropanol, DMSO, and denaturing chemicals such as urea and GnCl. Induro RT is more tolerant of all these common inhibitors and can still synthesize full-length cDNA in a reaction where they are present.
(4) Bypass activity prevents stalling at modified nucleotides
Cellular RNAs have many different modified ribonucleotides (psU, m6A, 5mC, 2′—-O-meU, mIA), which can cause the premature termination of cDNA synthesis. Bypass activity refers to the ability of the RT to perform continuous cDNA synthesis across these modified ribonucleotides without the RT stalling and resulting in a truncated cDNA product. A truncated cDNA can give biased coverage of the 5′ end of the sequence. An RT with good bypass activity will navigate the modification and generate full-length cDNA. Induro RT has the ability to bypass modified ribonucleotides more frequently than retroviral RTs.
In summary, while retroviral RTs are widely available and work well in many well-established protocols, intron-encoded RTs offer some advantages over retroviral RTs and can be a better choice for cDNA synthesis from long transcripts, RNAs with strong secondary structures, and RNA samples with inhibitors. Learn more about Induro Reverse Transcriptase.
NEB will not rent, sell or otherwise transfer your data to a third party for monetary consideration. See our Privacy Policy for details. View our Community Guidelines.
Don’t miss out on our latest NEBinspired blog releases!
- Sign up to receive our e-newsletter
- Download your favorite feed reader and subscribe to our RSS feed
Be a part of NEBinspired! Submit your idea to have it featured in our blog.


